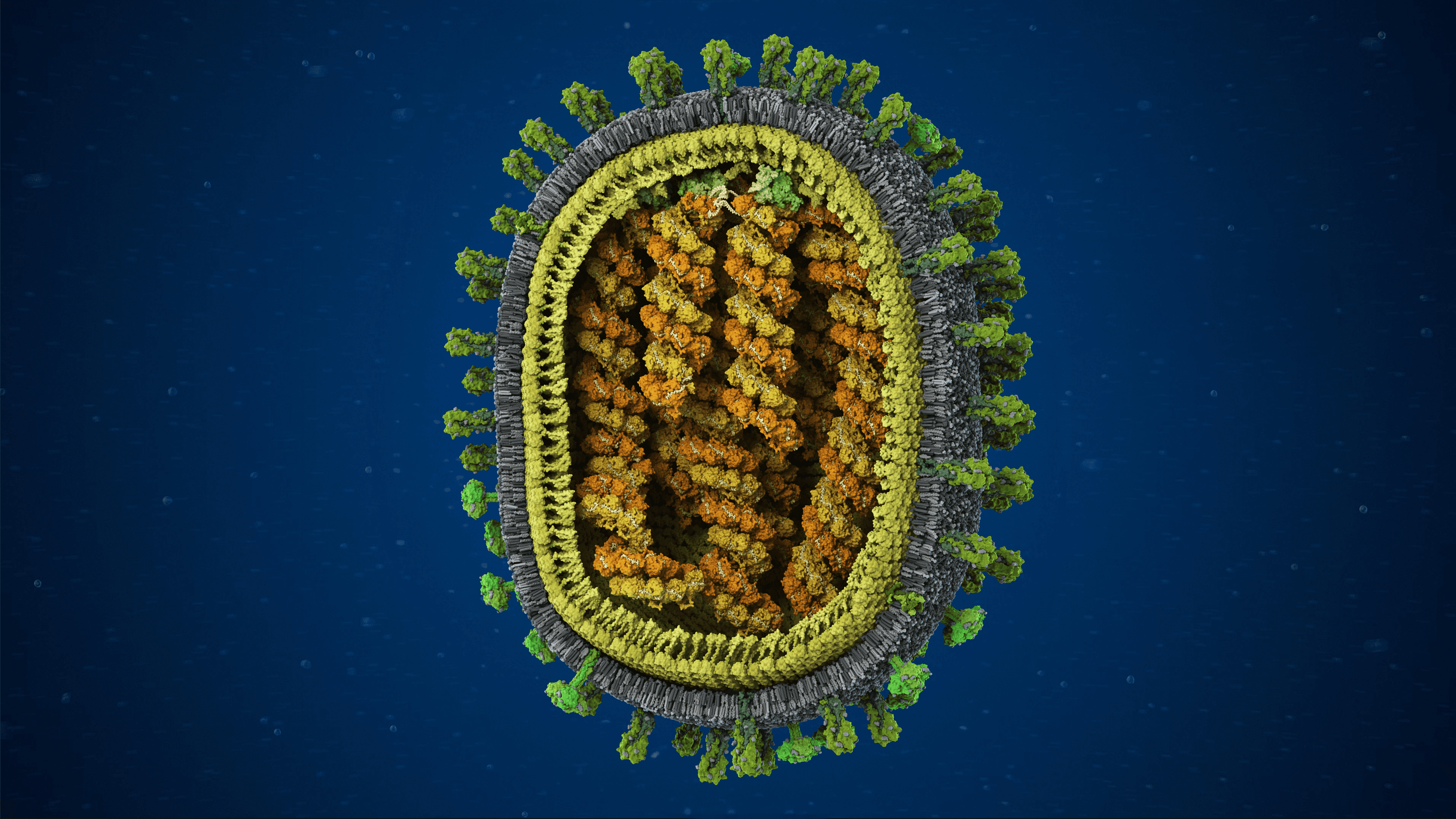
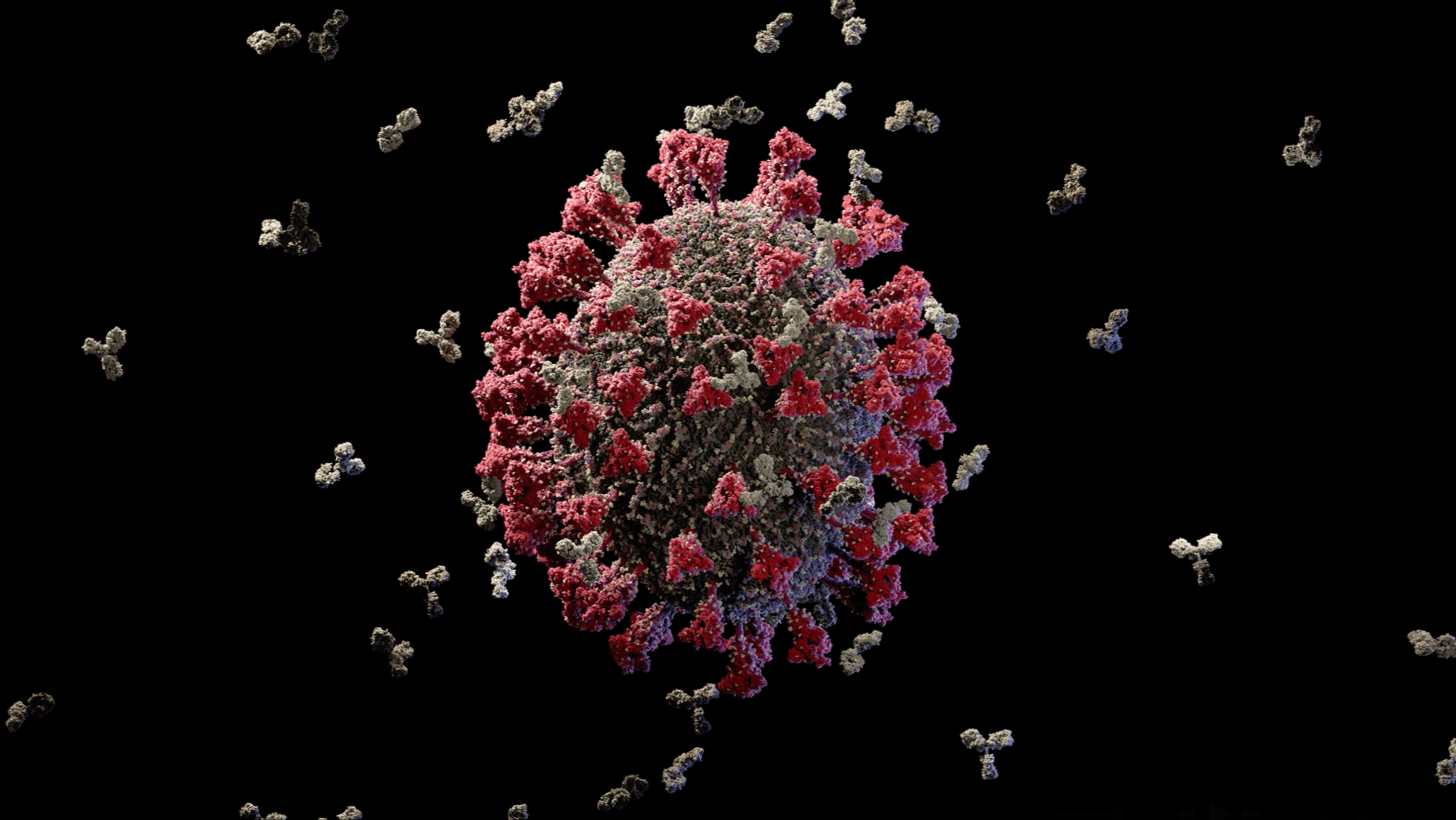Scientifically accurate model of the Zika virus
We created the first scientifically accurate atomic-resolution 3D model of the Zika virus, developed using state-of-the-art structural bioinformatics and the latest peer-reviewed research. Released during the height of the global Zika outbreak in 2016, the model offered an unprecedented view of the viral particle, revealing its molecular architecture with a level of detail not previously available.
We created the first scientifically accurate atomic-resolution 3D model of the Zika virus, developed using state-of-the-art structural bioinformatics and the latest peer-reviewed research. Released during the height of the global Zika outbreak in 2016, the model offered an unprecedented view of the viral particle, revealing its molecular architecture with a level of detail not previously available.
Project Goal
Project Goal
Project Goal
Project Goal
The goal of this project was to accurately represent the molecular structure of the Zika virus at atomic resolution, providing a reliable, research-driven visualization. At a time of widespread concern and limited public understanding of the virus, the model was intended to clarify how Zika is structured at the molecular level.
See full animation.
The goal of this project was to accurately represent the molecular structure of the Zika virus at atomic resolution, providing a reliable, research-driven visualization. At a time of widespread concern and limited public understanding of the virus, the model was intended to clarify how Zika is structured at the molecular level.
See full animation.
The goal of this project was to accurately represent the molecular structure of the Zika virus at atomic resolution, providing a reliable, research-driven visualization. At a time of widespread concern and limited public understanding of the virus, the model was intended to clarify how Zika is structured at the molecular level.
See full animation.
The goal of this project was to accurately represent the molecular structure of the Zika virus at atomic resolution, providing a reliable, research-driven visualization. At a time of widespread concern and limited public understanding of the virus, the model was intended to clarify how Zika is structured at the molecular level.
See full animation.
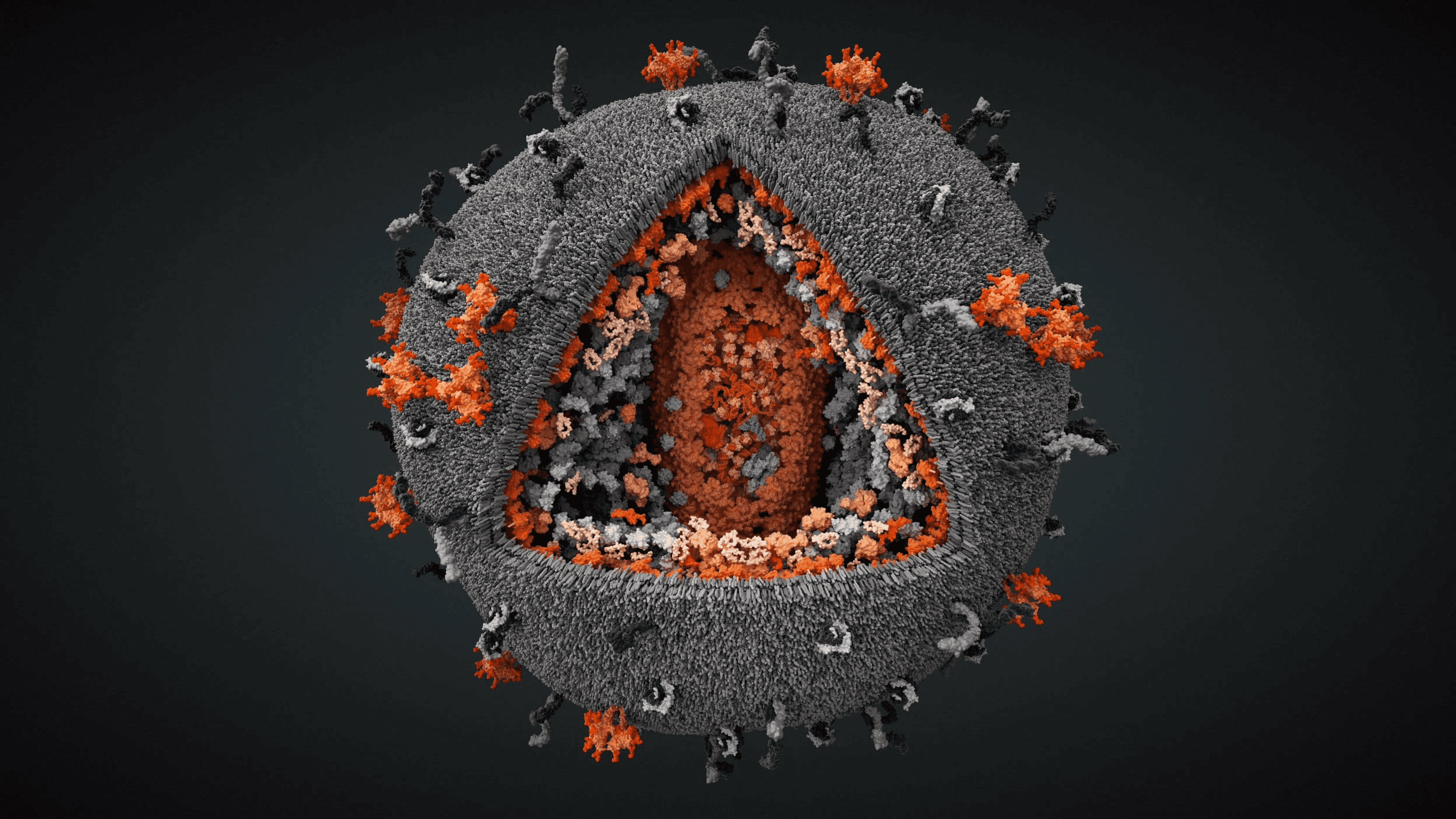
Zkia Virus with exposed core
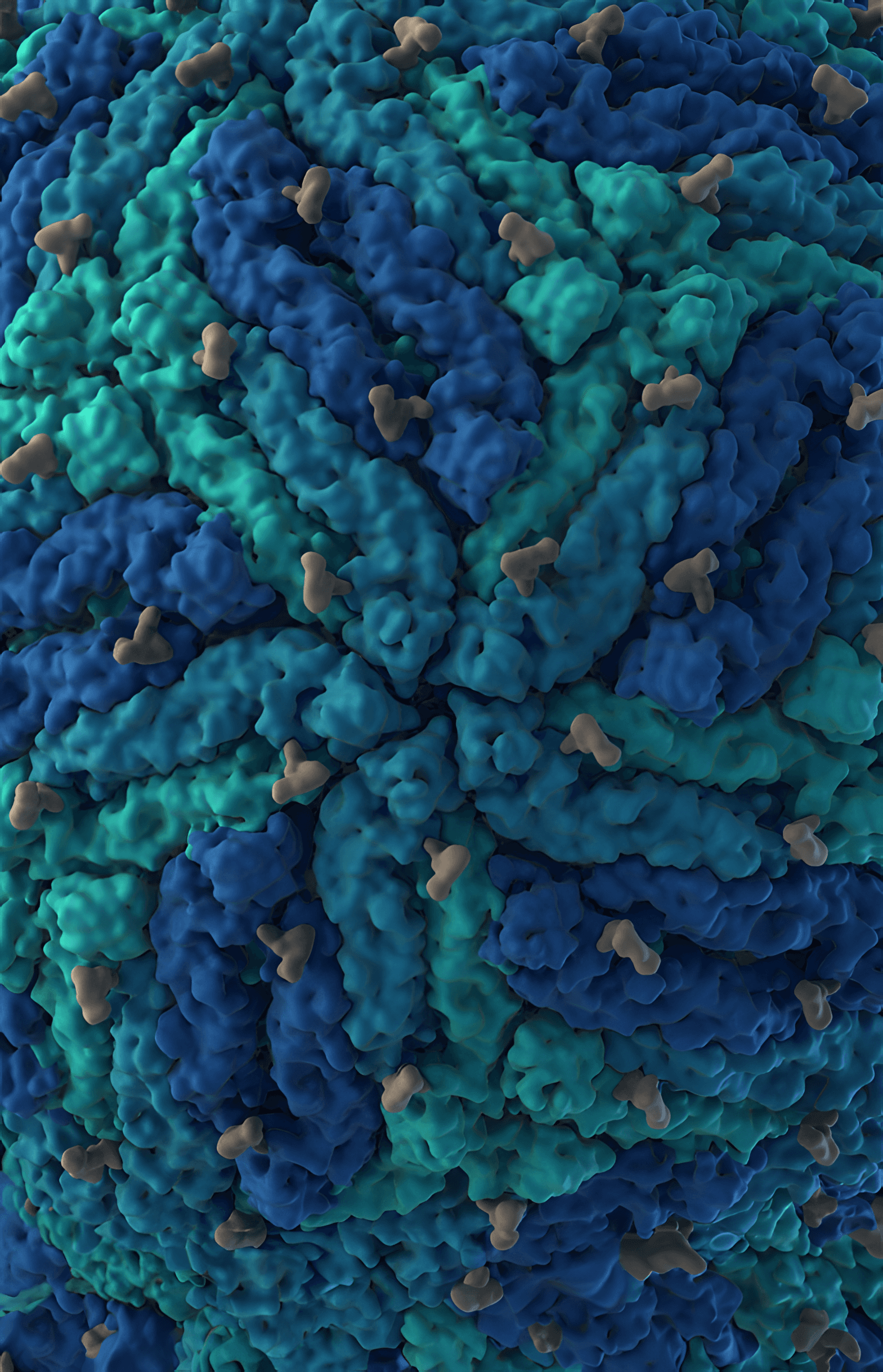

Lipid envelope with E and M proteins
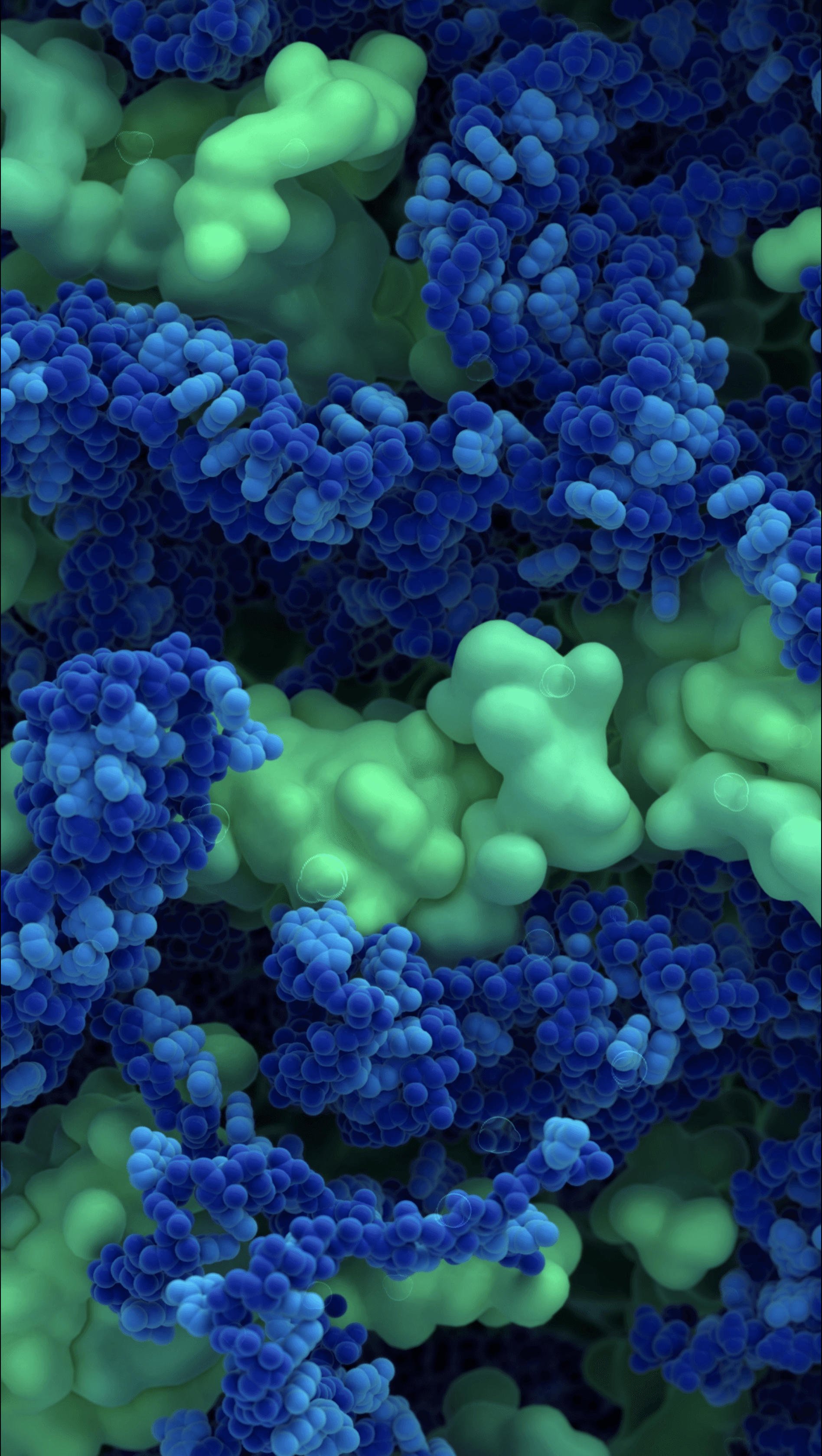

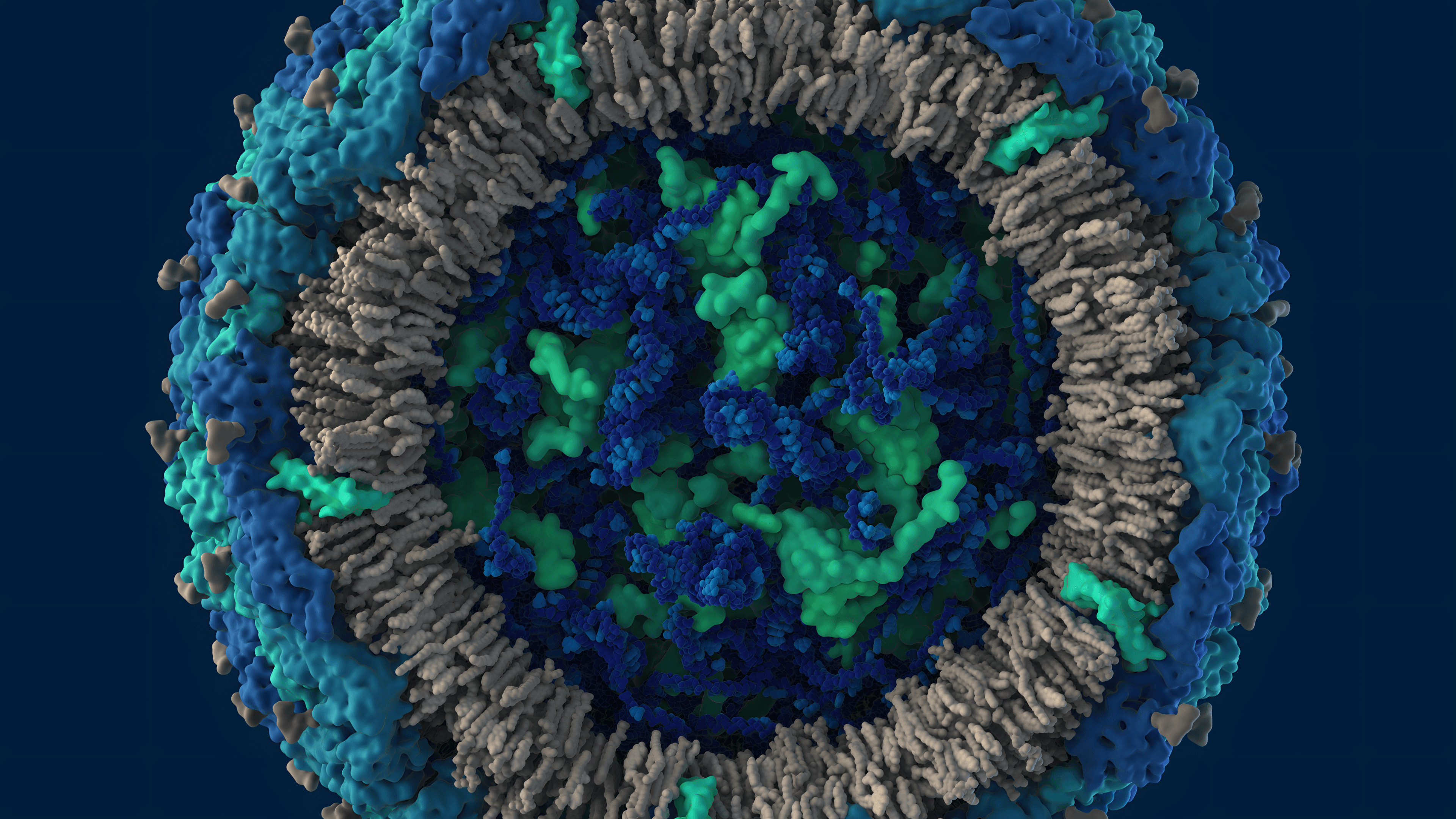
Capsid protein C and Genomic RNA
Capsid protein C and Genomic RNA
“As scientists work to unravel the mysteries of Zika, a scientific visualization firm has created a realistic 3D model of the mosquito-borne virus that lets you see it up close and extremely personally.”
“As scientists work to unravel the mysteries of Zika, a scientific visualization firm has created a realistic 3D model of the mosquito-borne virus that lets you see it up close and extremely personally.”
“As scientists work to unravel the mysteries of Zika, a scientific visualization firm has created a realistic 3D model of the mosquito-borne virus that lets you see it up close and extremely personally.”
“As scientists work to unravel the mysteries of Zika, a scientific visualization firm has created a realistic 3D model of the mosquito-borne virus that lets you see it up close and extremely personally.”




The Process
The Process
Animation type:
Animation type:
High-End 3D
High-End 3D
Project timline:
Project timline:
5 weeks
5 weeks
Team:










Find out which of our 26 scientific animation options works best for investor relations and communications:
The Zika virus model was produced using the same structural bioinformatics techniques employed in basic research and drug development. Protein structures, homology models, and comparative data from related viruses—such as Dengue, West Nile, and yellow fever—were integrated into a single coherent viral particle.
All components, including envelope proteins, lipid membranes, and internal structures, were assembled according to known biological constraints and validated structural data. Scientific accuracy guided every stage of production, with visual decisions driven by research evidence.
The Zika virus model was produced using the same structural bioinformatics techniques employed in basic research and drug development. Protein structures, homology models, and comparative data from related viruses—such as Dengue, West Nile, and yellow fever—were integrated into a single coherent viral particle.
All components, including envelope proteins, lipid membranes, and internal structures, were assembled according to known biological constraints and validated structural data. Scientific accuracy guided every stage of production, with visual decisions driven by research evidence.
Why did we use this
animation type?
Why did we use this
animation type?
Accurately conveying the molecular complexity of the Zika virus required a high-end 3D animation approach capable of representing scale, spatial relationships, and structural detail at atomic resolution.
Advanced 3D modeling and rendering allowed viewers to explore the virus from multiple perspectives while preserving scientific fidelity. This approach made it possible to present a visually compelling yet accurate representation suitable for both scientific audiences and mainstream media.
Accurately conveying the molecular complexity of the Zika virus required a high-end 3D animation approach capable of representing scale, spatial relationships, and structural detail at atomic resolution.
Advanced 3D modeling and rendering allowed viewers to explore the virus from multiple perspectives while preserving scientific fidelity. This approach made it possible to present a visually compelling yet accurate representation suitable for both scientific audiences and mainstream media.
Outcome:
Outcome:
The completed Zika virus model became one of the most widely referenced visualizations of the virus during the outbreak and was recognized as the most accurate representation of the Zika viral particle available at the time of release.
As part of Visual Science’s non-commercial Viral Park initiative, the project contributed to broader public and scientific engagement with virology and demonstrated the value of rigorous, high-fidelity molecular visualization during a global health crisis.
The completed Zika virus model became one of the most widely referenced visualizations of the virus during the outbreak and was recognized as the most accurate representation of the Zika viral particle available at the time of release.
As part of Visual Science’s non-commercial Viral Park initiative, the project contributed to broader public and scientific engagement with virology and demonstrated the value of rigorous, high-fidelity molecular visualization during a global health crisis.
Full animation
You may also want to see
Contact us
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
Contact us
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
10/10
“
Fantastic team to partner with throughout the entire process. Excellent output and management.

Global Marketing Director, Pfizer
10/10
“
We are very pleased with Visual Science — they are a very responsive group to work with and the final product is exactly what we had envisioned.”

CEO, Atsena Therapeutics
10/10
“
The scientific expertise really showed in the discussions and final products. Everybody was responsive and great to work with, and the animations were both engaging and accurate!”

Program Manager, American Chemical Society
10/10
“
Visual Science team has just the right blend of scientific acumen and digital animation expertise to pull off these challenging projects.”

CEO, Apton Biosystems
9/10
“
Visual Science is a fantastic partner, capable of rendering the most complex science in compelling ways. They understood the science, and their production was excellent.”

VP, Head of Communications, Scorpion Tx
7/10
“
The structural biology models from Visual Science are stunning! Visual Science implemented our guidelines into beautiful scientific imagery for our website and scientific slide decks.”

Science Content Lead, Dewpoint Tx
9/10
“
I was very impressed with scientific knowledge of the Visual Science team, which led to a fantastic visualization of the MOA of our drug. Attention to scientific details was extraordinary.”

Vice President, R&D, Unicycive Tx
10/10
“
We were impressed by the scientific sophistication of the Visual Science team and their skill in capturing the concepts we wanted to communicate. The final video was exceptional.”

CSO, Enterin
10/10
“
The quality of the work from Visual Science was outstanding. They provide a bespoke service that was responsive to our requests and I would recommend them highly.”

CEO, UNITY Biotechnology
10/10
“
Beautiful work that is detailed and thoughtful. Great final output.”

Sr. Comms & IR Associate, Candel Tx
10/10
“
We were impressed by the Visual Science team’s ability to use exact protein structures to depict complex mechanisms in <90 seconds. The visuals are beautiful and tell a clear story.”

President & CEO, ROME Tx
10/10
“
Excellent scientific partner and first class imagery. This team runs a tight ship and the process is clear. The tech and final product are first rate.”

VP, Corp Dev, Revolution Medicines
Contact us
Contact us
Visual Science is an award-winning medical animation and digital scientific communications company, trusted by leading biotech and pharmaceutical organizations since 2007, including J&J, Pfizer, Novartis, Roche, Takeda, Gilead, AbbVie, and 100+ others.
We specialize in science-grade MoA and MoD videos, medical animation, and scientific storytelling, as well as digital and AI-driven solutions for Medical Affairs, marketing, corporate communications, and investor relations.
© 2026, Visual Science. All rights reserved.
Visual Science is an award-winning medical animation and digital scientific communications company, trusted by leading biotech and pharmaceutical organizations since 2007, including J&J, Pfizer, Novartis, Roche, Takeda, Gilead, AbbVie, and 100+ others.
We specialize in science-grade MoA and MoD videos, medical animation, and scientific storytelling, as well as digital and AI-driven solutions for Medical Affairs, marketing, corporate communications, and investor relations.
© 2026, Visual Science. All rights reserved.
Visual Science is an award-winning medical animation and digital scientific communications company, trusted by leading biotech and pharmaceutical organizations since 2007, including J&J, Pfizer, Novartis, Roche, Takeda, Gilead, AbbVie, and 100+ others.
We specialize in science-grade MoA and MoD videos, medical animation, and scientific storytelling, as well as digital and AI-driven solutions for Medical Affairs, marketing, corporate communications, and investor relations.
© 2026, Visual Science. All rights reserved.
Visual Science is an award-winning medical animation and digital scientific communications company, trusted by leading biotech and pharmaceutical organizations since 2007, including J&J, Pfizer, Novartis, Roche, Takeda, Gilead, AbbVie, and 100+ others.
We specialize in science-grade MoA and MoD videos, medical animation, and scientific storytelling, as well as digital and AI-driven solutions for Medical Affairs, marketing, corporate communications, and investor relations.
© 2026, Visual Science. All rights reserved.